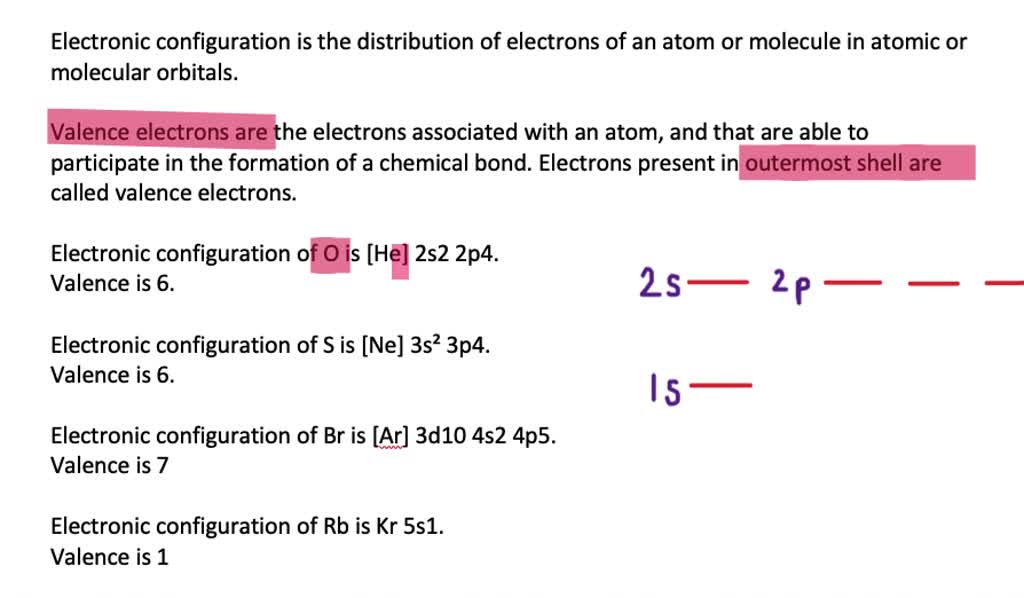
A bromine (Br) atom has 7 valence electrons. Valence electrons are the outermost electrons in an atom, which affect how atoms might react with one. See full answer below. Become a member. The BCl 3 Lewis structure is similar to BF 3 and BBr 3 since F and Br are in Group 7 and have 7 valence electrons. Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BCl 3 you can calculate the formal charges. You'll find the B in BCl 3 only has 6.
Drawing the Lewis Structure for BCl3
Viewing Notes: Macbook pro a1502 manual download.
- Br has 7 valence electrons and H has 1 valence electron, so that's 8 in total. You need 2 electrons for a bond so you end up with H bonded to Br and 6 electrons around Br. What has more valence.
- The amount of valence electrons in groups 3-12 cannot be predicted because their oxidation numbers are unknown. To find the number of valence electrons in groups 13-18, you take the series number and subtract 10 from it. Therefore, if Bromine is in series 17, 17-10 is 7, so Bromine has 7 valence electrons.

- The BCl3 Lewis structure is similar to BF3 and BBr3 since F and Br are in Group 7 and have 7 valence electrons.
- Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6).
- If you're not sure you have the best Lewis structure for BCl3 you can calculate the formal charges. You'll find the B in BCl3 only has 6 valence electrons.
- For the BCl3 Lewis structure there are a total of 24 valence electrons available.
See the Big List of Lewis Structures
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for BCl3. Boron has three valence electrons. Chlorine has 7, but since we have three of those we'll multiply them together. And we get a total of 24 valence electrons. Let's draw it. We'll put Boron at the center, and then let's put the Chlorines around it, there are three of them. And put some electrons. So we have 24. We'll put them between to form bonds, there's 6. And then on the outer atoms, 8, 10, 12, 14, 16, 18, 20, 22, 24. We've used all the valence electrons up.
The only problem is that Boron doesn't have an octet. The interesting thing is, Boron doesn't necessarily have to have 8 valence electrons. So I could draw it this way, but I could also draw it with a double bond. So to do the double bond over here, I'll just take these, get rid of those, and put them right here. And that double bond will give us 8 valence electrons for the Chlorine and 8 for the Bromine. The question is now, which of these structures is correct? To find out, what we can do is use the idea of formal charges. So to do that, we have an equation that'll help us.

So let's do the Chlorine, this one right here, and see what its formal charges are. We have 7 valence electrons for Chlorine on the periodic table. Nonbonding, these right here, we have 6. And then bonding, we have 2, and we'll put that over 2. Seven minus six minus one is zero. So the formal charge for this Chlorine is zero. For the Bromine here we have 3 valence electrons. Nonbonding, they're all bonding. And then bonding electrons: we have these, these, and these. So we have 8 over 2. So 3 minus 4 is negative 1. That's our formal charge on the Bromine. For the Chlorine right here, we have 7 minus 4 minus 4 over 2; so 7 - 4 - 2 equals +1. So the formal charge on this Chlorine is +1.
Now let's go over here. We have Chlorine, so that's going to be 7 minus 6 minus 2 over 2; that equals zero. So a formal charge of zero. For the Bromine here, we have 3 minus zero minus 6 over 2; 3 minus 3 gives us zero. Problem download chrome on macbook air. So the formal charge on the Bromine is zero. And since all these Chlorines are the same, they're all going to be zero as well.
Valence Electrons In Br-1
So when you look at formal charges, the molecule with the most zeros, closest to zero, that's the one that's going to be the best Lewis structure. And in this case, this one right here is the Lewis structure that's going to be most appropriate. This is Dr. B., and thanks for watching.
